 |
| Carnot Engine from Wikipedia |
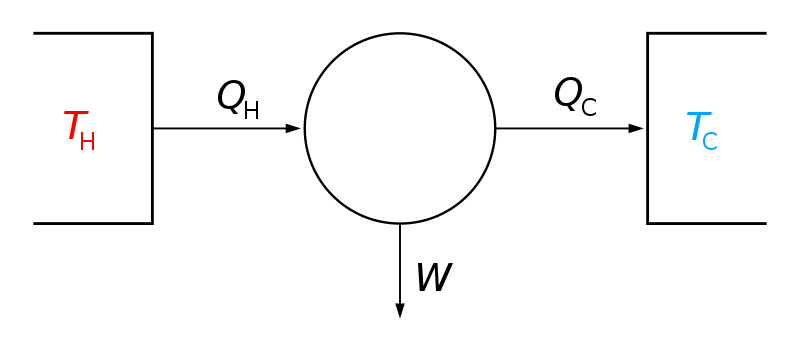
For some reason the simplest principles of thermodynamics baffle people. In any engine, there is all sorts of stuff going on inside. What matters most is what comes out compared to what goes in.
Nicholas Leonard Sadi Carnot was a French Military Engineer that knew that. To simplify a problem he developed the Carnot Engine. That simple drawing was too complicated for some to understand so it was simplified even more.
The Qn is the energy in, Qc is the energy out and W is the work. So Qh=Qc+W, If Qc=W, then only half of the energy is converted into usable work. Only half is pretty damn good really, your car engine is only about 0.35Qh. A modern gas turbine engine is close to 0.45Qc and if you add a cogeneration process like a steam turbine that uses the some of the 0.55Qh which is Qc, you can get more work, kicking the entire process up to 0.6Qh, or 60 percent efficiency.
 |
| Two Stage Carnot Heat Engine |
You can never get all the good out of Qh because of entropy, typically noted with the letter S. There is no perfect engine. With that in mind, any thermodynamic process can be simplified to some combination of Carnot Heat Engines, even the Earth's Atmosphere.
 |
| Atmosphere as a Carnot Heat Engine |
As all Red Neck Theoretical Physicists know the best place to start is at the beginning. For maximum entropy, W=Qc=S for each process and the whole process. If it doesn't, try to figure out why.
 |
| Atmosphere as a Carnot Heat Engine and some Initial Values to Play with |
Play with this simple model of a complex Atmospheric/Ocean system a see what you think, Physics is Phun!




No comments:
Post a Comment