 |
| The first day |
Using Wm-2 inside the liquid is not a perfect illustration of energy transfer. The energy transfer in water is typically in Joules per gram. For the ice at 0C the transfer to -1.9C would be closer to 8.4 Joules per gram instead of 9 Wm-2. The exact energy would depend on the salinity of the water and the purity of the ice.
For the first night, there is a blue bubble shown replacing the solar arrow. As the 350Wm-2 warm pool cooled, the water on the surface would start to freeze. Each gram of water that freezes would release 334 Joules per gram. Again, Joules per gram is not a perfect conversion for Wm-2, but it is pretty close.
The water would receive 350 Wm-2 during the day at as the surface began to freeze, would be limited to approximately 334Wm-2 until it sufficiently froze. That is only a gain of 16Wm-2, but because of the salinity of the water, could be as high as 25Wm-2, fresh ice is warmer than salt water at the freezing point. This creates a little Mexican standoff.
Rapidly cooling water has enough thermal inertia to freeze quickly. Slowly cooling water actually takes longer to freeze since it has to deal with the point of fusion energy standoff, known as the Mpemba Effect. The freezing of the surface tends to block convection from below reducing the rate of cooling. Warmer water with more energy can diffuse more readily allowing it to overcome the convection blockage. With a very small difference in energy, both the rate of convection and the rate of diffusion are minimized. The temperature inversion and the ambitious density of the salt water makes things even more entertaining.
The maximum density of fresh water is at the temperature of 4 C degrees. As water cools to that temperature is sinks slightly. In salt water, the impurities cause that maximum density temperature to disappear. The density is controlled by the salinity and as ice forms, some salt is expelled increasing the density causing the colder more dense water to sink and reverse of the more efficient convection path. This allows more energy to be retained than one would expect at first glance.
Another consideration with the faint sun is energy absorbed in the atmosphere. With little water vapor only ozone and oxygen would absorb any significant amount of incoming solar. Since clouds current reflect nearly 24% of the incoming solar an absorb nearly the same percentage of the solar not reflected, the faint sun's energy would be more efficiently transferred to the surface water. As long as the overall atmospheric composition is nearly the same, other than the water vapor content, energy would be transferred to the atmosphere both during 24 hour period by conduction. That is a small but not negligible energy transfer over a long enough period of time.
In addition to the small solar energy gain, there is another small geothermal energy input into the oceans that likely caused the liquid water to exist to begin with. Approximately 0.1 Wm-2 of energy are transferred to the oceans now. A younger Earth was more likely a more geologically unstable Earth. Even with a snowball Earth, there would likely be liquid water near the ocean floor with its huge pressures. With that liquid water, the energy available would be on the order of 307Wm-2, the freezing point of salt water. With the average solar energy available at the total surface area of 1/4 of the 700Wm-2 available at the top of the atmosphere and the weak absorbing atmosphere, there could be 175Wm-2 available at the surface with the 350Wm-2 available at the equatorial region. The Sun and the Moon would still have a gravitational effect causing water to flow and thinner ice to fracture. As long as there is a positive net gain in energy, the oceans would slowly warm, energy would be transferred to the young atmosphere and the globe would be warming.
The energy balance could end in a position like this. We have a warmer ocean with less energy. The solar in this drawing is based on 50% of 1361 Wm-2 available at the top of the atmosphere, 30% of that is reflected to space mainly by water vapor, 128Wm-2 is transferred to the atmosphere, absorbed by water and water vapor in the tropics and 345Wm-2 makes it to the surface. This drawing is close to current reality, the solar available in the tropics drives the ocean heat engine that warms the global. Global warming is why we are here to ponder global warming :) For global averages that would be 345/2=172.5 absorbed by the surface, 64Wm-2 absorbed by the atmosphere. There is only one major difference between this energy budget and the ones used in the Climate Change debate. This one does not include the Antarctic an other regions where there is no significant water vapor. Those regions are not in the moist air envelope that causes global warming and their climate never really changes.
More Stuff:
This is a plot of the specific enthaply of fresh water plus the 334 Joules required for the heat of fusion versus the ideal black body energy of an object of the same temperature. There is no such thing as an ideal black body and our oceans are not pure water, so some adjusting would be required for these two energy measures to be comparable. With most things though, it is not a bad idea to start with ideal relationships then work into the nitty gritty. By adding the 334Wm-2 for the heat of fusion I am attempting to determine an absolute enthalpy. Based on this ideal comparison, it would be easier for energy to leave the water, S-B than increase the absolute enthalpy above approximately 21 C degrees. Below 21 C, the absolute enthalpy is greater than the S-B ideal energy. According to S-B, the surface would still emit at its black body temperature but the emissivity of the surface is not fixed. The object does not have to emit perfectly because it is not a perfect object. Generally, the emissivity is assumed to be to be constant or at least the change assumed to be insignificant. That may be a valid assumption, but the heat of vaporization is also non-linear. Water evaporation at lower temperature due to partial pressure relationships have a higher energy per gram. That is still energy emitted, just not radiant energy. If all energy transfer is considered then there would be a true emissivity or effective emissivity.
So think of a glass of fresh water. Instead of thinking in temperature we think in terms of energy and density.
If the water in the glass contains more energy than 334.5Wm-2 it is less dense. If it contains less energy than 334.5Wm-2 it is also less dense. So in the glass water above the cute dark blue slice with the arrow would be colder, than light blue below the cute falling slice would have more energy than the bottom blue slice so the cute slice descends to meet up with its buddy with the same energy and density. Using the Stefan-Boltzmann relationship, the table lists the energy, the fixed energy of fusion, the ratio of the S-B to the fixed energy of fusion and the final column is the total enthalpy difference from the maximum density if the world were perfect. Even here, at 4C the enthalpy is not perfectly zero, there is 0.63 Watts or joules per some unit difference. The difference to the ideal freezing point of water is -18.27 units of energy. There is another factor though, the difference in density. The main variable that controls density is the force of gravity. On Earth, that force is determined by the gravitational acceleration constant of 9.81m/sec^2 at sea level. It could be one or ten, it has to be some odd ball number like 9.81. To simplify things I would change that sucker to 10, but then I would have to adjust all those numbers I slaved over a hot spread sheet to make. It would be nice though to have a simple calculation to determine the specific enthalpy of at least water that directly relates to the S-B energy.
The dynamics of water though make that a little more complicated. The speed of sound is a limit just like the speed of light is a limit for the Stefan-Bolzman equation which has this nifty little factor considered.
where:
- kB is the Boltzmann constant;
- h is the Planck constant;
- ħ is the reduced Planck constant;
- c is the speed of light in vacuum.
Because of the differences, in fluid dynamics you have different kinds of temperatures, Stagnation temperature, static temperature and there should be a dynamic temperature. All based on the ability of the fluid to transfer heat.
The stagnation temperature is the temperature at the zero velocity of a stream flow. For our cute little glass, the stagnation temperature would be at the ice barrier, no water flows through that barrier. Since I am working in energy, that point can be called the Stagnation Energy, which for pure water at our imperfect gravitational constant would be 316Wm-2 if the S-B number were perfect.
Static temperature is related to Stagnation temperature, for air,
where:
 static air temperature, SAT (kelvin or degree Rankine)
static air temperature, SAT (kelvin or degree Rankine) total air temperature, TAT (kelvin or degree Rankine)
total air temperature, TAT (kelvin or degree Rankine) Mach number
Mach number ratio of specific heats = approx 1.400 for dry air
ratio of specific heats = approx 1.400 for dry air
The T(total) is the stagnation temperature, instead of the speed of light, the equation considers the Mach number or the speed of sound which varies with density and composition of the media, in this case air, of the path of the energy.
So I could make my life simple and use every equation known to mankind, or tweak the S-B relationship to allow for changes in density that impact the rate of energy flow. This is actually the basis for the Relativistic Heat Conduction equations.
"The most interesting observation about RHC is that it reduces the second law of thermodynamics to a statement of the form
which is the “no action at a distance” principle of special relativity. Essentially, the RHC asserts that relativity and the second law of thermodynamics are two alternative, but equal statements about the nature of time. Both physical principles are mutually derivable from each other and are complementary.[1]"
Note: The C in RHC and the c in the S-B are not the same. RHC use C as the speed of second sound similar to the Mach number in fluid dynamics.



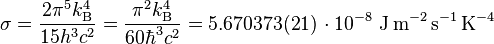


No comments:
Post a Comment